Pre-Series A Funding to Accelerate R&D of Cancer Early Detection Blood Test
Cosomil, Inc. (Headquartered in Bunkyo-ku, Tokyo; CEO: Yu Kagami) has secured funding from prominent investors including Coral Capital, ANRI, and Greencore K.K. This infusion of capital will significantly expedite the advancement of our R&D efforts focused on early-stage disease diagnostics, leveraging our proprietary technology. Additionally, it will drive the expansion of our operations internationally.

As a pioneering startup, we are dedicated to the widespread application of our “Single-molecule Enzyme Activity-based Liquid Biopsy” technology, a breakthrough resulting from collaborative research between the University of Tokyo and RIKEN. Aligned with our overarching vision of “Creating a world without disease and people living life to the fullest,” we are actively engaged in the development of early-stage diagnostics for various diseases, while also providing R&D support to healthcare companies.
■ Single-molecule Enzyme Activity-based Liquid Biopsy Technology
This groundbreaking technology represents a form of “liquid biopsy” testing, aligning with the global trend in recent years to intricately diagnose diseases through the analysis of biomolecular information in bodily fluids, collected in a minimally invasive manner.
Distinguished by its precision, our technology holds significant promise for early disease detection. Its exceptional accuracy allows for the identification of subtle changes at the single molecule level, focusing on evaluating enzyme activity, which is more proximate to phenotype than gene and protein levels (Science Advances, 2020, 6, eaay0888). The capability to conduct the assay with as little as 1 µL of blood not only underscores the technology’s effectiveness but also positions it as a compelling option for at-home testing.
Beyond disease diagnosis, we are actively engaged in collaborative efforts with pharmaceutical companies and other partners to explore new enzyme activity biomarkers. This exploration extends the application of our technology to areas such as drug efficacy evaluation and patient stratification, opening avenues for impactful contributions to the broader landscape of healthcare innovation.
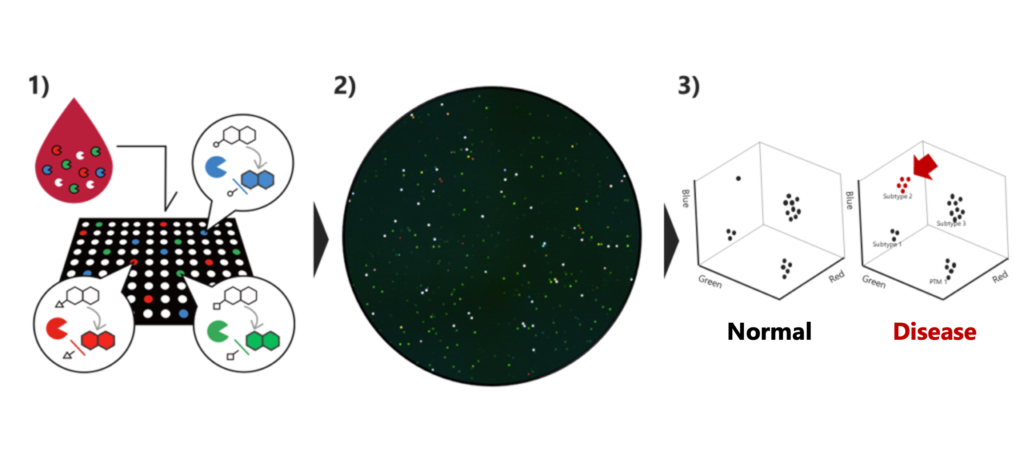
■ Pancreatic cancer early risk test to be launched in 2024
In the context of Japan’s rapidly aging population, where nearly one in every two individuals is expected to face a cancer diagnosis, the significance of early detection cannot be overstated. While the survival rates for cancer are notably higher with early intervention, detecting pancreatic cancer at an early stage poses a unique challenge due to the absence of subjective symptoms. In fact, only about 10% of patients can undergo surgery upon detection (Lancet, 2011, 378, 607-620).
Our revolutionary liquid biopsy testing technology offers a solution to this challenge, enabling the early detection of pancreatic cancer. Having conducted extensive clinical research involving over 600 blood samples from multiple medical institutions, our technology has demonstrated exceptional sensitivity and specificity in detecting stage 0-II early-stage pancreatic cancer. Building upon these promising results, we are on track to introduce a minimally invasive pancreatic cancer risk test to the market in 2024. Notably, our test surpasses conventional tumor marker tests in terms of sensitivity and cancer type specificity for early-stage cancer. This advancement is expected to facilitate early detection, streamlining the need for exhaustive examinations of organs beyond the pancreas. To further validate and expand the application of our technology, we are poised to conduct additional clinical studies with the goal of obtaining regulatory approval in both Japan and the United States.
The funds raised through this strategic financing round will be pivotal in expediting the clinical and business development of our pancreatic cancer test agents. Moreover, we aim to intensify our research endeavors toward the creation of early diagnostic agents for a spectrum of cancers and non-cancerous diseases, alongside the development of user-friendly home testing kits.
■ Comment from CEO: Yu Kagami, Ph.D
Under the leadership of our CTO Shingo, who courageously became the University of Tokyo’s first pharmaceutical student to launch a business based on his research, we are delighted by the significant progress of our venture. This success owes its gratitude to the support of numerous individuals, including investors, seasoned entrepreneurs, physicians, and experts, and is fundamentally rooted in the tireless dedication of our diverse team. Beyond our core of researchers, our team has evolved to include clinical technologists, engineers, and business developers, reflecting a multidisciplinary approach to innovation. With recent funding, we’ll advance Japanese technology globally, striving for a world free from disease. Thanks to all contributors, excited for the positive impact ahead.
